-
-
欧盟CE认证
EU CE CERTIFICATION
- 2021-02-01
-
-
- 2020-05-28
- 2020-05-28
- 2020-05-28
- 2020-05-28
- 2020-05-28
- 2020-05-28
国际认证
INTERNATIONAL CERTIFICATION
-
- 2020-05-28
- 2020-05-28
- 2020-05-28
- 2020-05-28
- 2021-11-25
-
-
-
-
专业认证 用心服务
信誉第一,服务领先!
全国服务热线:
021-34122669
Medical devices
Regulation (EU) 2017/745
|
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC |
Summary list of titles and references of harmonised standards
The summary below consolidates the references of harmonised standards published by the Commission in the Official Journal of the European Union (OJ). It reproduces information already published in the L series of the OJ. It contains all references which, when the summary was generated, still provided a presumption of conformity together with references already withdrawn from the OJ.
The Commission services provide this summary for information purposes only. Although they take every possible precaution to ensure that the summary is updated regularly and is correct, errors may occur and the summary may not be complete at a certain point in time. The summary does not as such generate legal effects.
Regulation (EU) 2017/745 on medical devices - Summary list as pdf document
Document date: 05/01/2022 - Created by GROW.H.3 - Publication date: n/a - Last update: 27/01/2022
Download links:欧盟官网下载链接
https://ec.europa.eu/docsroom/documents/48579
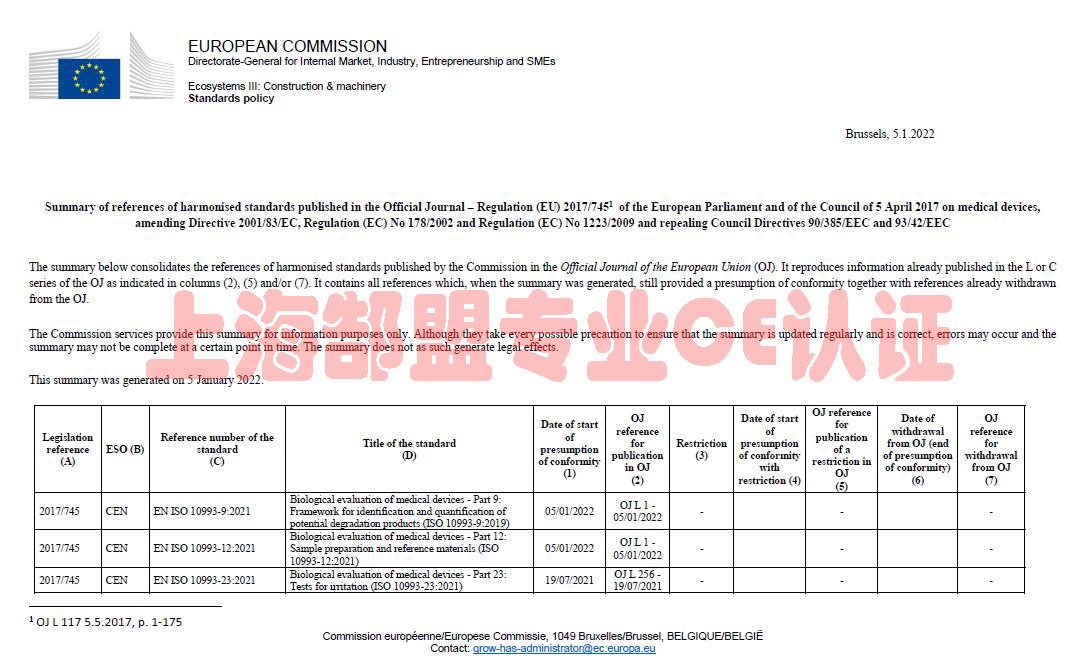
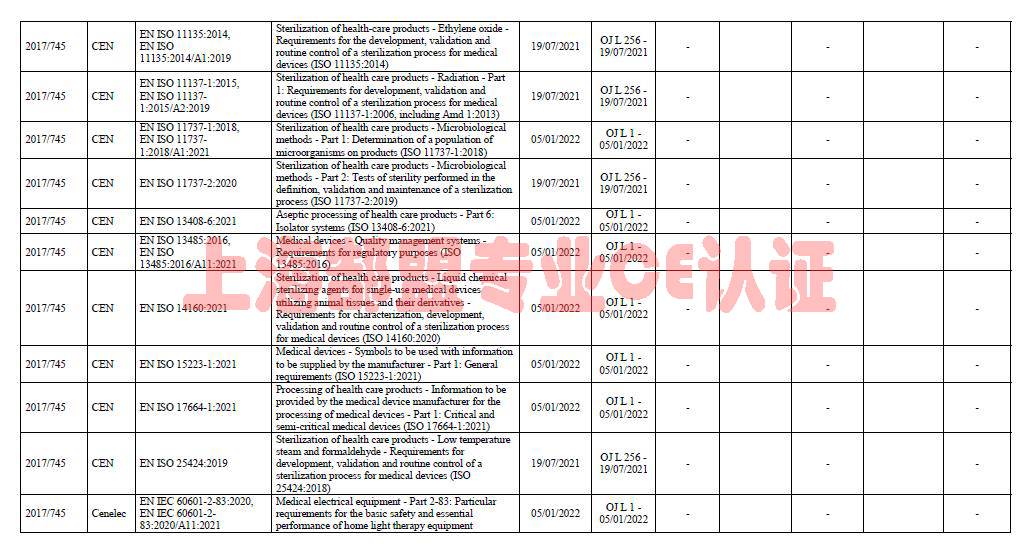
医疗器械CE认证协调标准清单-MDR标准清单-欧盟官网
联系我们
友情链接:
新闻资讯
客户服务
郜盟服务
关于我们
版权所有 All Rights Reserved.
021-34126062
















